We use cookies to make your experience better. To comply with the new e-Privacy directive, we need to ask for your consent to set the cookies. Learn more.
PRIME-XV Hematopoietic Cell Basal XSFM
Xeno-free, serum-free basal medium for human hematopoietic progenitor cell culture
Hematopoietic cells are often obtained from bone marrow, peripheral blood, and umbilical cord blood, albeit in very limited numbers. The ability to expand hematopoietic progenitor populations can be difficult to achieve. A successful expansion must provide sufficient numbers of fully functional hematopoietic progenitor cells that are able to self-renew and differentiate into all functional blood cells.
Provides Optimal Performance
Xeno-free, serum-free basal medium for human hematopoietic progenitor cell culture
- Supports maintenance of CD34+ hematopoietic stem and progenitor cell populations
- Optimized expansion of hematopoietic stem and progenitor cell populations
- Maintains balanced lineage differentiation potential
Designed and manufactured to facilitate transfer from research to clinic. Supported by robust raw material controls and supply chain management
- FDA, Federal, and State registered - cGMP-compliant manufacture
- EN ISO 13485:2016 certified
- MDSAP certified
- Extensive QC testing including functionality, sterility (USP <71>), and endotoxin (USP <85>)
- Drug Master Files (DMFs) filed with the FDA – available upon request
Custom sizes and packaging available upon request.
Contact us for international pricing and custom formulations.
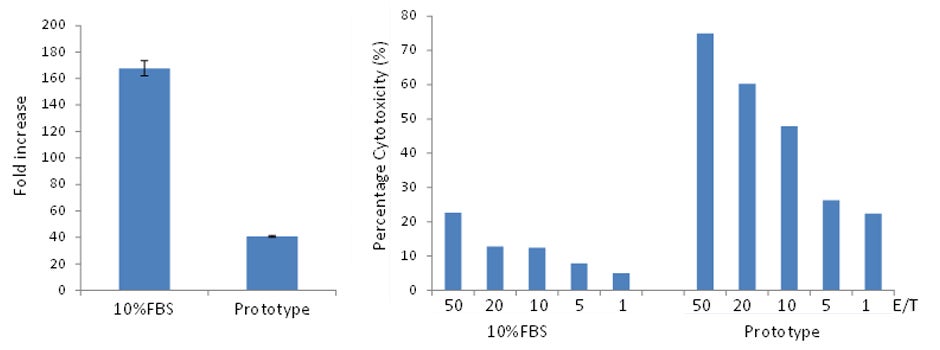

Figure 1 CD34+ hematopoietic progenitor cells derived from human cord blood were cultured in PRIME-XV Hematopoietic Cell Basal XSFM or a commercially-available xeno-free expansion medium, both supplemented with a cocktail of cytokines (TPO, SCF, FLT-3L, IL-3 and IL-6). After 3, 7 and 9 days, the TNC (A) and fold expansion (B) of CD34+ cells were quantified.