We use cookies to make your experience better. To comply with the new e-Privacy directive, we need to ask for your consent to set the cookies. Learn more.
PRIME-XV T Cell CDM
Chemically defined and animal component-free medium for T cell culture. PRIME-XV T Cell CDM supports viable T cell expansion across different culture vessels.
PRIME-XV T Cell CDM is the first commercially-available, chemically defined, animal component-free medium for the expansion and transduction of human T cells. The formulation is optimized to deliver consistently vigorous growth while maintaining T cell functionality and potency.
Provides Optimal Performance
Chemically defined, animal component-free T cell culture medium
- Supports T cell expansion in static and dynamic automation systems while maintaining functionality
- Eliminates the adverse effects undefined components cause on T cell phenotypes
- Supports polarization to targeted T cell types, such as Th1, Th2, cytotoxic T cells, to further possible therapy applications
- Performs transduction of T cells without requiring spinoculation or transduction enhancers
Designed and manufactured to facilitate transfer from research to clinic. Supported by robust raw material controls and supply chain management:
- FDA, Federal, and State registered - cGMP-compliant manufacture
- EN ISO 13485:2016 certified
- MDSAP certified
- Extensive QC testing including functionality, sterility (USP <71>), endotoxin (USP <85>), and mycoplasma (USP <63>)
- Drug Master Files (DMFs) filed with the FDA – available upon request
Custom sizes and packaging available upon request.
Contact us for international pricing and custom formulations.
PRIME-XV T Cell CDM supports viable T cell expansion across different culture vessels
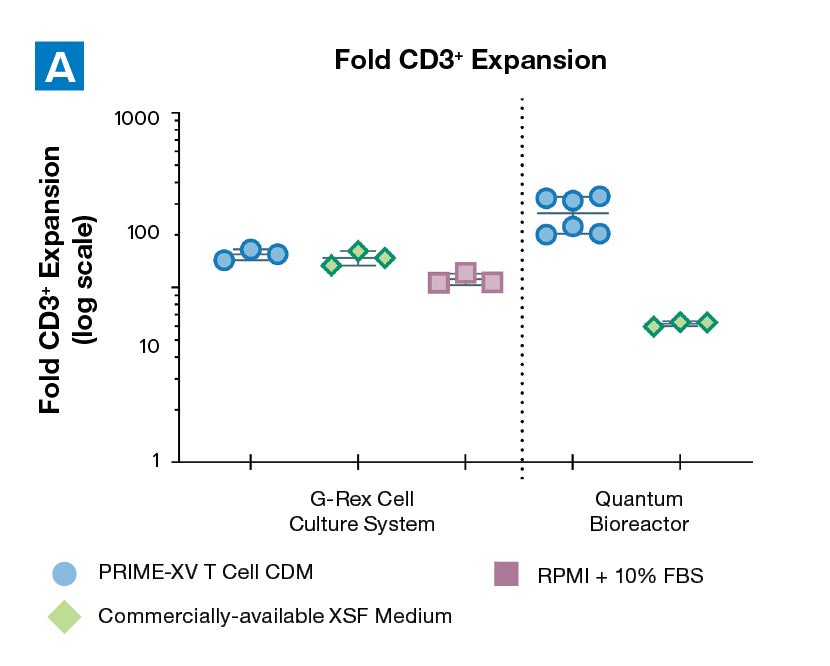

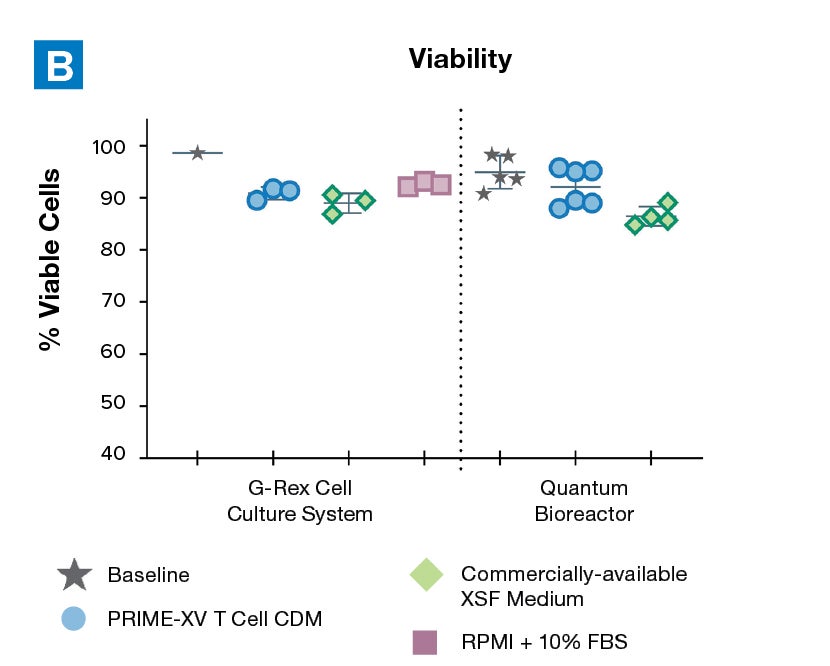

Figure 1: (A) Fold expansion of CD3+ cells in PRIME-XV T Cell CDM is robust across the 3 documented culture systems, performing as well as or better than FBS-supplemented RPMI and commercially-available XSF media. (B) Viability is maintained at or above 85% by the end of the culture period in all 3 cell culture systems. Though significant inter-donor variability is observed, these data are representative of 3 donors, analyzed on day 14 (G-Rex) or day 9 (Quantum Bioreactors).
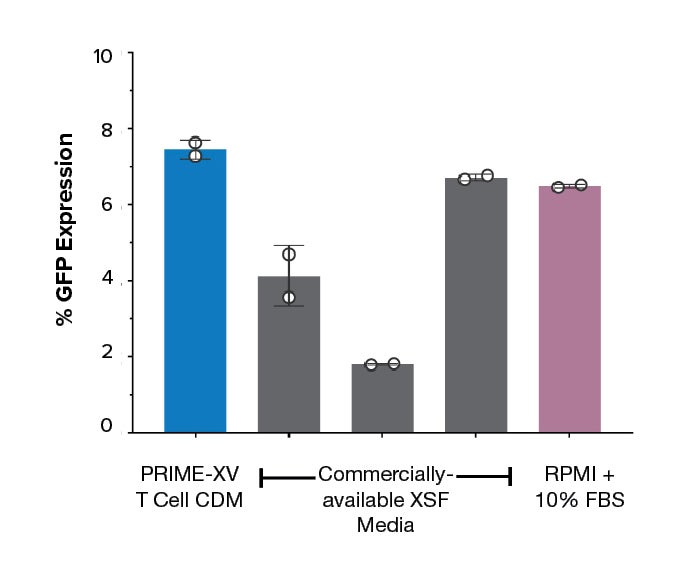

Figure 2: Enhancer-free transduction in PRIME-XV T Cell CDM is equal to or better than commercially-available xeno-free media and FBS-supplemented RPMI. Transduction efficiency of GFP reporter-positive cells at day 14 of expansion are robust in the chemically defined medium, even in the absence of transduction enhancers.
Publications, presentations, and protocols available for download
- Protocols support G-Rex and Quantum
- Protocols for plates and flasks can be provided upon request.