We use cookies to make your experience better. To comply with the new e-Privacy directive, we need to ask for your consent to set the cookies. Learn more.
CTGrade GMP rh IL-10
CTGrade GMP rh IL-10 is a recombinant human protein that is produced from E. coli and is designed to support basic, translational, and clinical research, as well as commercial applications, and offers:
- High biological activity verified by a relevant bioactivity assay
- Low endotoxin levels
- ≥ 97% purity
- High lot-to-lot consistency
Size: 50 µg, 100 µg, and 1 mg. Custom sizes and packaging available on request.
IL-10 is an anti-inflammatory cytokine produced by macrophages and type 2 T helper (Th2) cells.
- Inhibits the production of pro-inflammatory cytokines such as interferon gamma (IFNγ), tumor necrosis factor alpha (TNFα), interleukin 2 (IL-2), interleukin 3 (IL-3), interleukin 4 (IL-4), and granulocyte-macrophage colony-stimulating factor (GM-CSF), made by macrophages and regulatory T cells
- Suppresses antigen presentation on antigen presenting cells, and enhances the survival, proliferation, and antibody production of B cells
The biological activity of CTGrade GMP interleukins and growth factors is standardized, where applicable, to WHO International standards, providing cell and gene therapy developers consistent, lot-to-lot biological activity and performance.
The CTGrade GMP products are manufactured in a facility that does not use or process beta- lactam containing materials. No animal- or human-derived materials were used during manufacturing or as ingredients. These products are manufactured, tested, and released in an ISO 9001:2015 certified facility following cGMP practices. USP chapter <1043> for ancillary materials has been considered in the manufacture of these products.
SDS-PAGE Showing a Single Prominent Band at 18.8 kDa
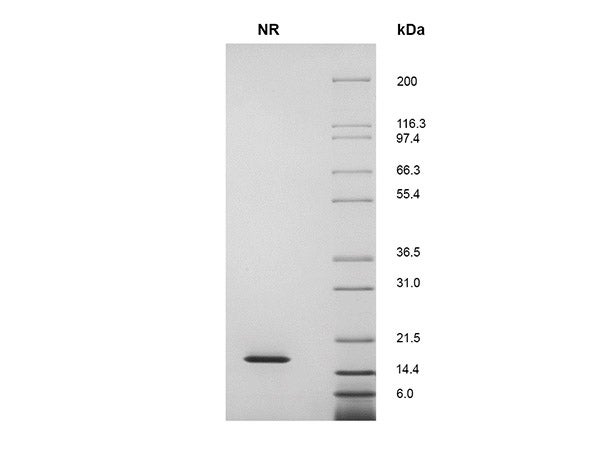

Figure 1. CTGrade GMP rh IL-10 (1 μg) was resolved with SDS-PAGE under non-reducing (NR) conditions and visualized by InstantBlue staining showing a single prominent band at 18.8 kDa.
Cell-Based Proliferation Assay
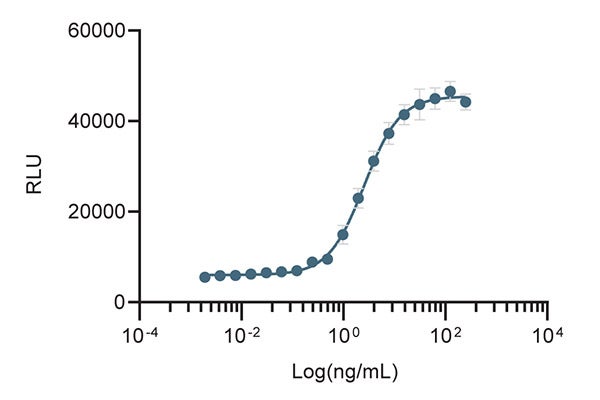

Figure 2. The biological activity of CTGrade GMP rh IL-10 was determined in a cell proliferation assay using MC/9 mouse mast cells.
Technical Specifications
| Alternate Names | B-TCGF, CSIF, TGIF |
| Accession Number | P22301 |
| AA Sequence | MSPGQGTQSE NSCTHFPGNL PNMLRDLRDA FSRVKTFFQM KDQLDNLLLK ESLLEDFKGY LGCQALSEMI QFYLEEVMPQ AENQDPDIKA HVNSLGENLK TLRLRLRRCH RFLPCENKSK AVEQVKNAFN KLQEKGIYKA MSEFDIFINY IEAYMTMKIR N |
| Predicted Molecular Mass | Non-covalent homodimer, 18.8 kDa (161aa) |
| Formulation | CTGrade GMP rh IL-10 lyophilized at 1 mg/ml in 10 mM Sodium Phosphate, pH 7.5, 0.2 µm filtered |
| Protein Purity | ≥ 97% determined by reducing and non-reducing SDS-PAGE analysis |
| Endotoxin | < 0.05 EU/µg using USP <85> / EP 2.6.14 |
| Bioactivity | ED50 is determined by the dose-dependent proliferation of MC/9 cells. The ED50 is typically less than 5 ng/mL. The international units of CTGrade GMP rh IL-10 is approximately 5.0 x 103 IU/µg, which is calibrated against recombinant human interleukin 10 WHO International Standard (NIBSC code 93/722). |
| Quality | Carrier-free and no animal- or human-derived materials were used during manufacturing |
*For research use or further manufacturing and in ex vivo cell therapy applications.
**Animal component-free to the tertiary level (no animal-origin components or sub-components are used in the product, raw materials, including packaging or the manufacturing process). Supporting documentation available upon request.
***Lot-specific values for the following specifications are supplied with each product on its corresponding COA. The values provided here are minimum expected values to pass internal requirements.







