We use cookies to make your experience better. To comply with the new e-Privacy directive, we need to ask for your consent to set the cookies. Learn more.
CTGrade GMP rh IL-21
CTGrade GMP rh IL-21 is a recombinant human protein that is produced from E. coli and is designed to support basic, translational, and clinical research, as well as commercial applications, and offers:
- High biological activity verified by a relevant bioactivity assay
- Low endotoxin levels
- ≥ 97% purity
- High lot-to-lot consistency
Size: 50 µg, 100 µg, and 1 mg. Custom sizes and packaging available on request.
IL-21 is a common chain cytokine regulating many cell types of the immune system. The IL-21 receptor (IL-21R) activates the JAK/STAT signaling pathway and is expressed on T, B, and natural killer (NK) cells.
- Plays an important role in the development of humoral immunity through its effects on B cell biology including differentiation, affinity maturation, and memory responses
- Regulates T cell responses including survival of CD8+ T cells and inhibition of Treg cells. IL-21 plays a pivotal role in Hodgkin’s lymphoma and suppresses contact hypersensitivity reactions
- Promotes a less differentiated phenotype of CAR T cells while promoting the effector functions of the engineered cells
The biological activity of CTGrade GMP interleukins and growth factors is standardized, where applicable, to WHO International standards, providing cell and gene therapy developers consistent, lot-to-lot biological activity and performance.
The CTGrade GMP products are manufactured in a facility that does not use or process beta- lactam containing materials. No animal- or human-derived materials were used during manufacturing or as ingredients. These products are manufactured, tested, and released in an ISO 9001:2015 certified facility following cGMP practices. USP chapter <1043> for ancillary materials has been considered in the manufacture of these products.
SDS-PAGE Showing a Single Prominent Band at 15.4 kDa


Figure 1. CTGrade GMP rh IL-21 (1 μg) was resolved with SDS-PAGE under non-reducing (NR) and reducing (R) conditions and visualized by InstantBlue staining showing a single prominent band at 15.4 kDa.
Cell-Based Proliferation Assay
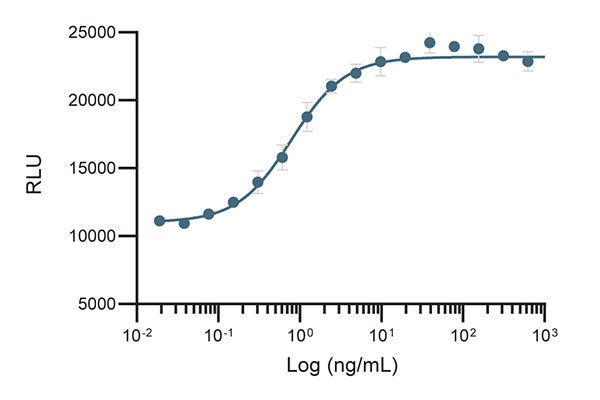

Figure 2. The biological activity of CTGrade GMP rh IL-21 was determined in a B9 cell-based proliferation assay.
Technical Specifications***
| Accession Number | Q9HBE4 |
| AA Sequence | MQDRHMIRMR QLIDIVDQLK NYVNDLVPEF LPAPEDVETN CEWSAFSCFQ KAQLKSANTG NNERIINVSI KKLKRKPPST NAGRRQKHRL TCPSCDSYEK KPPKEFLERF KSLLQKMIHQ HLSSRTHGSE DS |
| Predicted Molecular Mass | Monomer, 15.4 kDa (132 aa) |
| Formulation | CTGrade rh IL-21 lyophilized at 1 mg/ml in 10 mM Sodium Phosphate, pH 7.5, 0.2 µm filtered |
| Protein Purity | ≥ 97% determined by reducing and non-reducing SDS-PAGE analysis |
| Endotoxin | < 0.05 EU/µg using USP <85> / EP 2.6.14 |
| Bioactivity | ED50 is determined by the dose-dependent proliferation of B9 cells. The ED50 is typically less than 5 ng/mL. Activity is > 1.1 x 106 U/mg, when calibrated against internal standard IL-21 measured in cell proliferation assay using B9 cells. |
| Quality | Carrier-free and no animal- or human-derived materials were used during manufacturing |
*Animal component-free to the tertiary level (no animal components or sub-components are used in the product, raw materials, including packaging or the manufacturing process). Supporting documentation available upon request.
**For research use or further manufacturing and in ex vivo cell therapy applications.
***Lot-specific values for the following specifications are supplied with each product on its corresponding COA. The values provided here are minimum expected values to pass internal requirements.







