We use cookies to make your experience better. To comply with the new e-Privacy directive, we need to ask for your consent to set the cookies. Learn more.
Quality Media by Design

Current Good Manufacturing Practices (cGMP) guidelines are regulations provided by the United States Food and Drug Administration (FDA) to ensure the safety and efficacy of products manufactured by food, pharmaceutical, and medical device companies. The FDA permits flexibility in its interpretation relative to each particular product, leaving room for the manufacturer to determine the most appropriate and effective means for implementing the regulations. The primary intent of the regulations is to provide guidelines to ensure the proper design, monitoring and control of the manufacturing processes. This includes the requirement for a robust Quality System Management (QSM) to manage the manufacturing process from the beginning, starting with the qualification of raw material suppliers to the eventual release of the product. A strong QSM with oversight from Quality Assurance (QA) during the development and manufacturing of the product(s) is required to prevent deviations, contaminations, or product failures.
At Irvine Scientific, the goal set during the development of new products is to improve upon benchmark media while maintaining consistent, high-level performance within all four of its product lines, which include: Industrial Cell Culture (ICC), Cell Therapy (CT), Assisted Reproductive Technologies (ART) and Cytogenetics (Cyto). As an example, two new ART media products recently launched for use in In Vitro Fertilization (IVF), Continuous Single Culture-NX (CSCM-NX) and Continuous Single Culture-NX Complete (CSCM-NXC), were developed using Irvine Scientific’s first-generation single-step media, Continuous Single Culture (CSCM) and Continuous Single Culture Complete (CSCM-C) as benchmarks. CSCM and CSCM-C were originally launched back in 2012 and were the first and only single-step culture media on the market at the time to support the culture of human embryos in an uninterrupted environment without the need to refresh media or change dishes. The idea of uninterrupted culture was to minimize stress to the embryo and recreate a more “natural” environment to improve clinical outcomes. Keeping this in mind, the next-generation media (CSCM-NX and CSCM-NXC) were designed with a lower lactate concentration to minimize metabolic stress on the embryo while improving on blastocyst rates. The design control put in place required the use of raw materials from Irvine Scientific’s cGMP inventory which were all qualified by the QA Department. A robust raw material program and supply chain ensured the highest quality materials were incorporated into the products in compliance with cGMP regulations. This strong raw material program is one example of how to ensure the development of high-quality products with consistent lot to lot performance. Below are a few highlights from Irvine Scientific’s raw material handling program implemented to adhere to cGMP regulations in the development of all of its products, particularly the most recently launched CSCM-NX and Continuous CSCM-NXC:
Dual Sourcing of Raw Materials
Dual sourcing translates to lower risk, mitigates out-of-stock situations, and improves inventory management. Whenever possible, at least two qualified vendors are approved for each raw material.
Sourcing of the Highest Grade Materials
High compendia grade components assure the highest purity of the raw material and consistency of the final product.
Customer-Specific Raw Material Requirements
For customer-specific requests, a system is in place to support custom raw material sourcing and/or specifications. Irvine Scientific takes a flexible and collaborative approach in custom raw material control to meet customer needs.
Continuous Vendor Improvement
On-going, continuous, and pro-active quality monitoring of vendors is practiced, which allows control and advanced preparation in informing customers with change notifications.
MEGA Assay
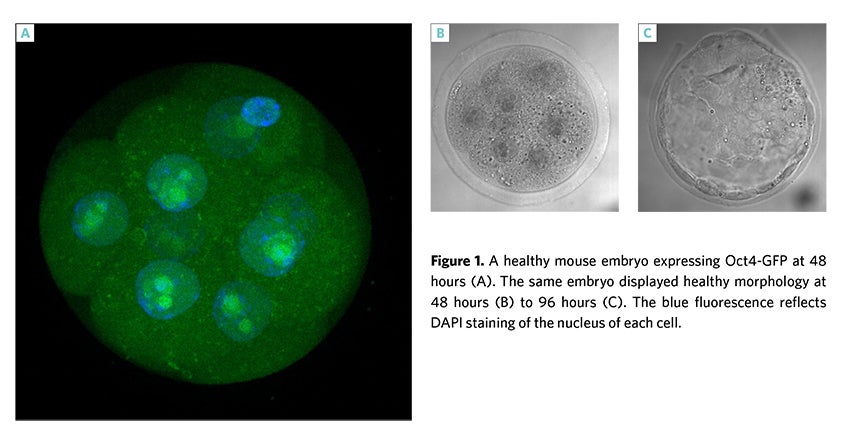
This bioassay was developed by Irvine Scientific which has increased sensitivity to embryotoxic materials providing more information about the development of the embryo at an earlier stage than the traditional mouse embryo assay (MEA). The MEGA Assay uses mouse embryos that are harvested from transgenic mice that express green fluorescent protein (GFP) under the control of the endogenous OCT4 promoter, which is a critical early marker of normal embryo development. This assay has been incorporated into the release criteria of some of our critical raw materials as an added level of assurance to our testing (Figure 1).
In addition to the raw materials, another important factor to ensure lot to lot consistency is the monitoring of the processes and equipment utilized during manufacturing. All products from Irvine Scientific with a sterility claim, including CSCM-NX and CSCM-NXC, are filled using validated aseptic processes that are revalidated for sterile filling approximately every six months in accordance with cGMP regulations. Regular validation of all equipment utilized during manufacturing in accordance with regulations also ensures the quality consistency of its products. Irvine Scientific’s Quality Control (QC) laboratory also closely monitors the environment and performs in process product testing throughout the manufacturing process until final release testing. Each lot of product is required to meet all test specifications at the time of final release prior to being placed into the general inventory. For example, each lot of CSCM-NX and CSCM-NXC must meet the specifications in Table 1 before prior to release by the QA Department for sale to customers:
Table 1: Final Release Criteria for CSCM-NX and CSCM-NXC
| TEST | CSCM-NX / CSCM-NXC |
|---|---|
| Sterility | Pass |
| pH (at 37°C, 5% CO2) | 7.3-7.5 |
| Osmality | 260-270 mOsm/Kg H2O |
| Endotoxin | ≥ 0.25 EU/mL |
| Mouse Embryo Assay | ≥ 80% of control |
| Human Sperm Survival Assay | ≥ 70% of control |
As an FDA registered company, Irvine Scientific follows 21CFR820 Quality Systems Regulations and is regularly inspected by the FDA. And with nearly five decades in the cell culture business and more than fifty 510(k)s, more than thirty CE marked products have been released which are distributed worldwide.
References:
1. Quality System (QS) Regulation/Medical Device Good Manufacturing Practices. https://www.fda.gov/MedicalDevices/DeviceRegulationandGuidance/PostmarketRequirements/QualitySystemsRegulations/
2. Facts About the Current Good Manufacturing Practices (CGMPs). https://www.fda.gov/drugs/developmentapprovalprocess/manufacturing/ucm169105.htm
3. Current Good Manufacturing Practices (CGMPs): https://www.fda.gov/food/guidanceregulation/cgmp/
5. Genetic mouse embryo assay: improving performance and quality testing for assisted reproductive technology (ART) with a functional bioassay. Reprod Biol Endocrinol. 2016; 14: 13. Published online 2016 Mar 24.








