We use cookies to make your experience better. To comply with the new e-Privacy directive, we need to ask for your consent to set the cookies. Learn more.
Protocol for Mesenchymal Stem Cell Isolation
Mesenchymal stem cells (MSCs) have the ability to modulate the immune system and are multipotent, which allow these adult stem cells to be valuable therapeutic candidates. Furthermore, MSCs can readily be obtained from different sources, including the umbilical cord, cord blood, adipose tissue, and bone marrow. The following protocols have been adapted and simplified based on published reports1,2 and have not been validated by FUJIFILM Irvine Scientific. This should be used for reference only. For other MSC-related protocols and related reagents, see here.
Materials
For MSC Isolation from Adipose Tissue
- Blood/saline solution containing adipose tissue
- 40% Dulbecco’s Modified Eagle Medium (FUJIFILM Irvine Scientific, Catalog #9031) with Fetal Bovine Serum
- Six-well plates
- Phosphate Buffered Saline (PBS) (FUJIFILM Irvine Scientific, Catalog #9235)
- 100µM nylon mesh
- 40µM nylon mesh
- Collagenase
- NH4Cl
For MSC Isolation from Cord Blood
- Cord Blood
- RPMI Medium 1640 (FUJIFILM Irvine Scientific, Catalog #9161)
- Ficoll-PaqueTM Premium
- Culture Medium
- Phosphate Buffered Saline
For MSC Isolation from Umbilical Cord
- Umbilical Cords
- Hypochlorite Solution
- Phosphate Buffered Saline
- 10% FBS/DMEM-low glucose
- Collagenase
- Culture Medium
Protocols
The following protocols are for mesenchymal stem cell isolation:
Mesenchymal Stem Cell Isolation from Adipose Tissue – Rapid Isolation
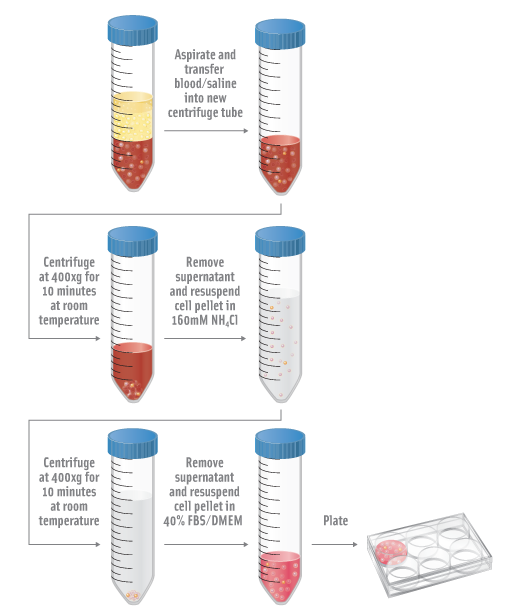
- Aspirate blood/saline into a 50mL conical tube.
- Centrifuge at 400xg for 10 minutes at room temperatures.
- Resuspend pellet in 160mM NH4Cl for 5 minutes at room temperature.
- Centrifuge at 400xg for 10 minutes at room temperature.
- Remove supernatant and resuspend pellet in 40% FBS/DMEM and plate.
- Incubate at 37°C, 5% CO2 incubator overnight.

Mesenchymal Stem Cell Isolation from Adipose Tissue – Standard Isolation
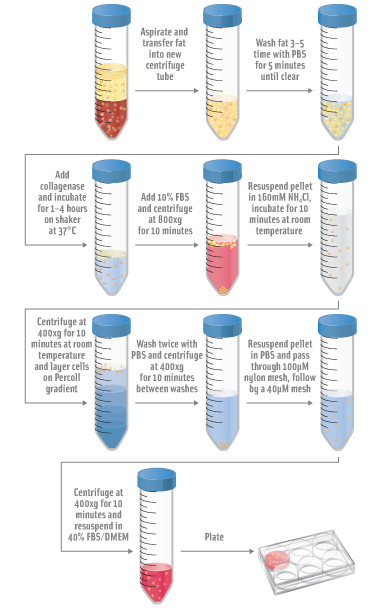
- Aspirate off saline and oil phases.
- Wash ~250mL of fat 3-5 times for 5 minutes in PBS each wash, discarding lower phase until clear.
- Add collagenase and incubate 1-4 hours at 37°C on a shaker
- Add 10% FBS to neutralize collagenase.
- Centrifuge digested fat at 800xg for 10 minutes.
- Aspirate floating adipocytes, lipids and liquid, leaving stromal vascular fraction (SVF) pellet.
- Resuspend SVF pellet in 160mM NH4Cl and incubate for 10 minutes at room temperatures.
- Centrifuge at 400xg for 10 minutes at room temperatures.
- Layer cells on Percoll or Histopaque gradient.
- Centrifuge at 1000xg for 30 minutes at room temperature.
- Wash cells twice with PBS and centrifuge at 400xg for 10 minutes between each wash.
- Resuspend cell pellet in PBS and filter cells through 100µM nylon mesh.
- Pass cells through 400µM mesh.
- Centrifuge at 400xg for 10 minutes.
- Resuspend cell pellet in 40% FBS/DMEM and plate.
- Incubate at 37°C, 5% CO2 incubator overnight.

Mesenchymal Stem Cell Isolation from Cord Blood
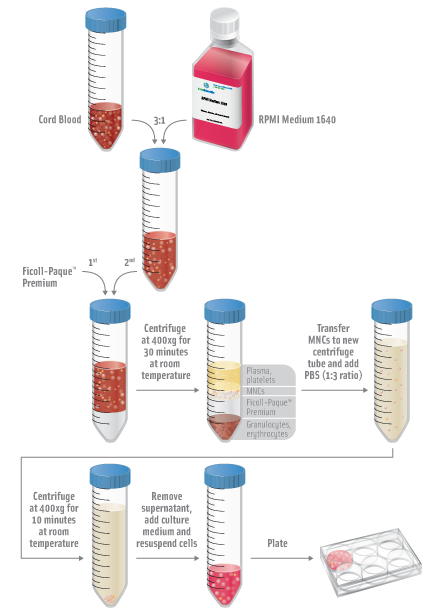
- Dilute cord blood with RPMI Medium 1640 with a 3:1 ratio (3 parts cord blood to one part RPMI).
- Isolate mononuclear cells (MNCs) by density gradient centrifugation at 400xg for 30 minutes at room temperature using Ficoll-Paque™ Premium according to the manufacturer’s instructions.
- Transfer MNCs to new centrifuge tube and add PBS with a 1:3 ratio (1 part MNCs to 3 parts PBS).
- Centrifuge at 400xg for 10 minutes at room temperature.
- Remove supernatant and resuspend cells by adding culture medium and plate.
- Incubate at 37°C, 5% CO2 incubator overnight.
*Ficoll-Paque™ is a registered trademark of GE Healthcare

Mesenchymal Stem Cell Isolation from Umbilical Cord
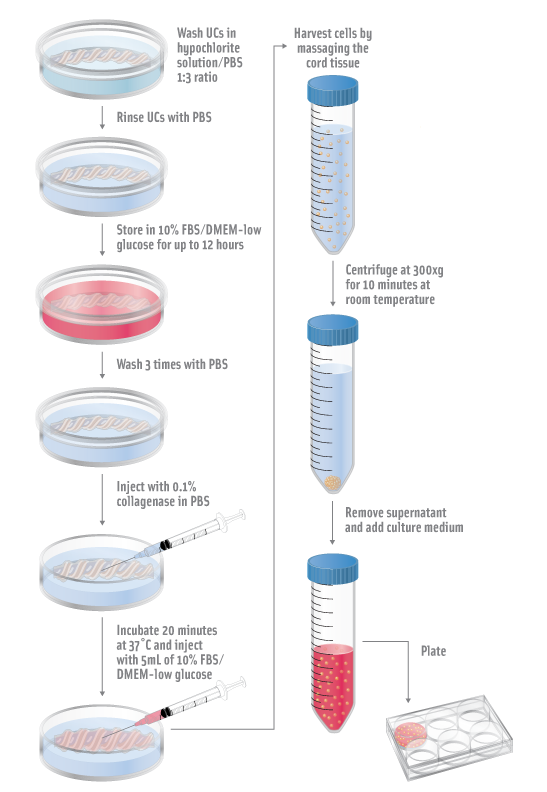
- Wash umbilical cords (UCs) in a hypochlorite solution (1:3).
- Rinse UCs with PBS.
- Store UCs in 10% FBS/DMEM-low glucose for up to 12 hours.
- Wash UCs three times with PBS.
- Inject vein and arteries with 3mL 0.1% collagenase in PBS.
- Incubate for 20 minutes at 37°C.
- Inject 5mL DMEM-low glucose with 10% FBS.
- Harvest cells by massaging the cord tissue.
- Centrifuge at 300xg for 10 minutes at room temperatures.
- Remove supernatant, add culture medium and plate.
- Incubate at 37°C, 5% CO2 incubator overnight.

Related Protocols:
References:
(1) Francis MP, Sachs PC, Elmore LW et al. Isolating adipose-derived mesenchymal stem cells from lipoaspirate blood and saline fraction. Organogenesis 6: 11-14, 2010
(2) Secco M Zucconi E, Zatz M et al. Multipotent stem cells from umbilical cord: cord is richer than blood! Stem Cells 26:146-50, 2008

