We use cookies to make your experience better. To comply with the new e-Privacy directive, we need to ask for your consent to set the cookies. Learn more.
Media Design for the Fed-Batch Production of Antibodies
Effective production of therapeutic antibodies requires optimal cell growth for peak yields. Efficiently maximizing antibody production typically requires a cell growth curve with a rapid rise to maximal viable cell density that plateaus for an extended period of time. To obtain this growth profile, an optimal cell medium and culture strategy tailored to each cell line and antibody pair is needed. The most common culture strategies are batch and fed-batch cultures. In batch cultures, cells are grown in an initial volume of media throughout the entire culture process. Conversely, in fed-batch cultures, the initial media volume is supplemented with concentrated nutrients during the process. Fed-batch systems have significant advantages over batch systems such as higher cell densities and more concentrated antibody protein titers.1 Determining the optimal growth medium and feed combination for fed-batch antibody production requires surveying through different media pairs.
One common method for medium and feed optimization is a step-wise approach that first determines the best candidate growth medium in batch culture based primarily on cell growth. This best-performing growth medium is subsequently used to evaluate a panel of feeds in fed-batch cultures. A major drawback to this approach is the possibility of prematurely eliminating a growth medium that performs extremely well in combination with a feed medium. By not including the other growth media during the screening of feed, the top-performing fed-batch media pair may likely be missed for an underperforming, less synergistic pair. For this reason, we at Irvine Scientific recommend moving forward with a ‘paired approach’ by evaluating a collection of top growth medium with different feeds in fed-batch cultures. This approach ensures that a fuller range of potentially synergistic combinations are successfully captured and can be subsequently evaluated for other parameters such as antibody titer and product quality. The following media-development case study was conducted at Irvine Scientific and demonstrates the specific benefits and advantages of a paired-media evaluation.
To compare a step-wise and paired approach, Chinese hamster ovary (CHO) cells were first grown in batch mode in various growth media and evaluated for viable cell density, percent viability, and antibody titer (Figure 1).
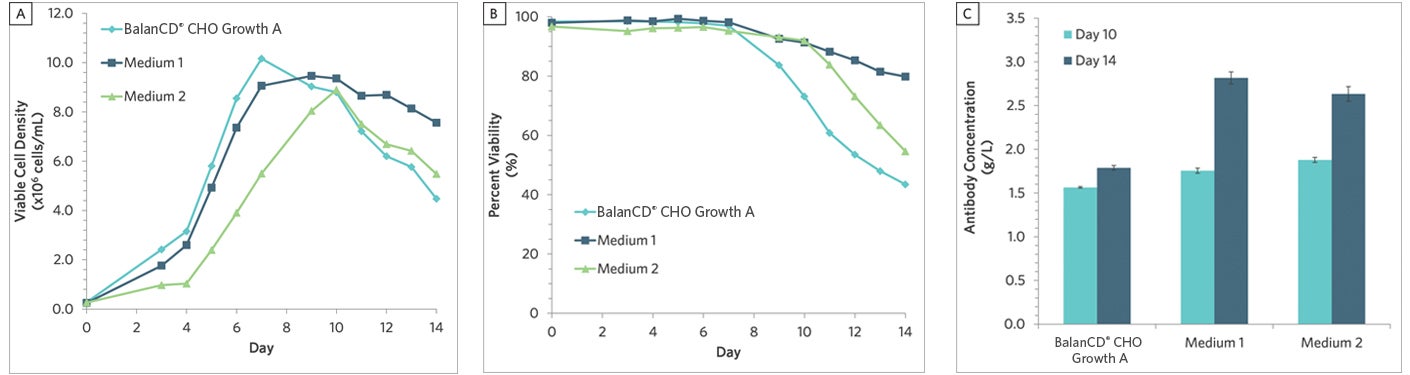
Figure 1. Batch culture of CHO cells grown in various growth media and evaluated for (A) viable cell density, (B) percent viability, and (C) antibody concentration. Data is presented as the mean or mean ± SD (n = 2). Medium 1 had the highest viable cell density, percent viability, and antibody concentration at the end of the 14-day batch culture. In a typical step-wise approach, only Medium 1 would move on to the screening of feed media.
Through day 8, BalanCD CHO Growth A medium had the highest viable cell density but rapidly fell to the lowest viable cell density by the end of the 14-day batch culture. Meanwhile, Medium 1 had the highest viable cell density, percent viability, and antibody titer at the end of the batch culture. In a step-wise media approach, Medium 1 would move on for further feed evaluation while both BalanCD CHO Growth A and Medium 2 would be excluded since they did not perform as well. However, we recommend moving forward in a paired approach with at least a few different growth media to better investigate more growth media and feed combinations.
The importance of a paired media survey is most apparent when evaluating the fed-batch cultures. In this assessment, all 3 growth media were each paired with 5 different feeds. Addition of feed occurred every other day from days 3 to 11 and the fed-batch cultures were evaluated for cumulative cell density, antibody concentration, viable cell density, and percent viability (Figure 2).
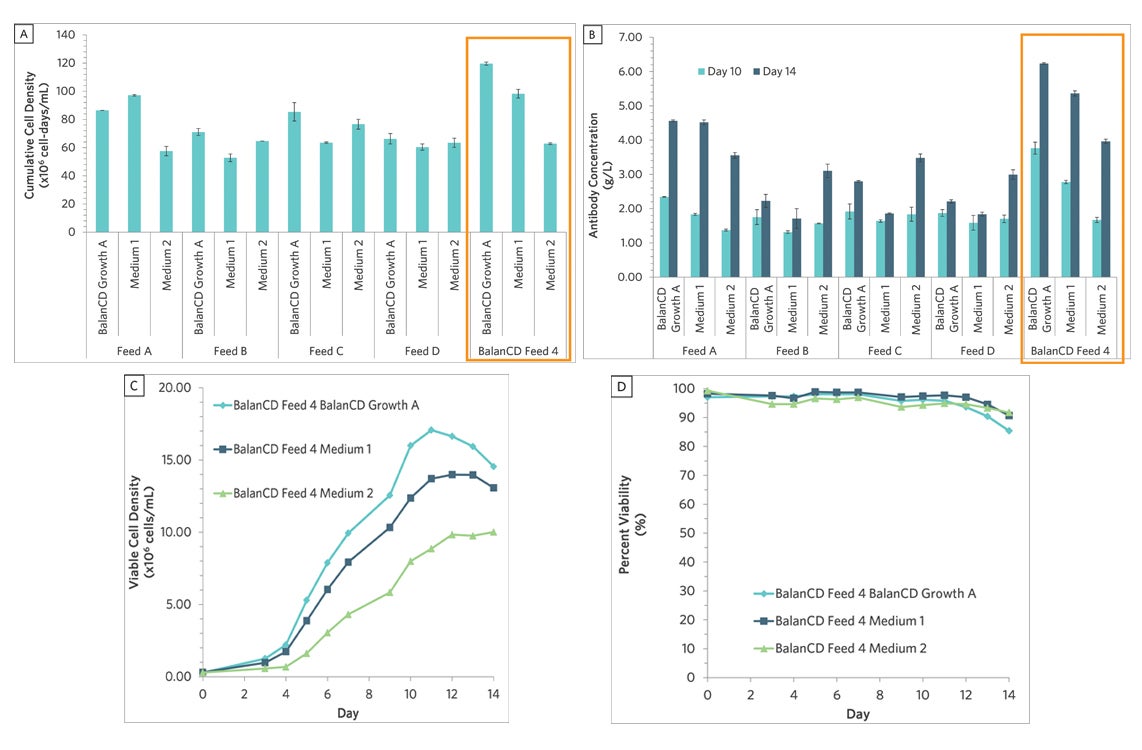
Figure 2. Fed-batch culture of CHO cells grown in all 3 growth media with different feeds and evaluated for (A) cumulative cell density, (B) antibody concentration, (C) viable cell density, and (D) percent viability. Data is presented as the mean or mean ± SD (n = 2). The combination of BalanCD CHO Growth A with BalanCD CHO Feed 4 produced the highest cell densities and antibody titer among all the candidate media combinations. This synergistic effect would have been missed if only Medium 1 moved forward to the fed-batch evaluation.
While Medium 1 performed the best in the initial batch cultures, it was not the best performer in combination with any of the 5 feeds. Instead, BalanCD CHO Growth A with BalanCD CHO Feed 4 outperformed all the other growth and feed media combinations to have the highest antibody titer and cell densities. These results highlight the importance of paired media screens which test more combinations of media to ensure the discovery and capture of the highest synergistic effects. In a conventional step-wise approach, the top-performing combination would have been missed since BalanCD CHO Growth A would be eliminated after the batch culture evaluation.
Another important aspect of the antibody production process is a balance between high titers and antibody product quality. Though maximizing antibody titer is an important parameter in the production process, antibodies still require an appropriate glycosylation profile to ensure excellent product quality. Since antibody glycosylation depends on the available nutrients and culture conditions, a culture mainly focused on cell growth may have extraordinarily high titer with poor glycosylation and ineffective antibodies.5,6 Thus, it is critical to assess antibody product quality after establishing the top-performing growth and feed media. If antibody product quality is marginally compromised, further media optimization can be performed to regain antibody efficacy. However, if antibody product quality is drastically decreased, the selection of growth or feed media would have to be re-evaluated. With a step-wise approach to media optimization, this may result in repeating the fed-batch culture studies with the second-best candidate growth media. In contrast, with a paired approach, a collection of antibodies from a variety of growth and feed media pairs is ready to be evaluated for product quality. A paired media approach ensures a higher probability in finding the top-performing media but also readily provides a number of alternative media choices. To obtain efficient, cost-effective manufacturing and effective antibody product, an appropriate balance between cell growth and antibody product quality must be found.
Furthermore, the chosen growth and feed media from paired evaluation can be further improved based on the spent media analysis indicating clone-specific metabolic behaviors. Cells typically use glucose and glutamine as their primary energy sources.2 However, excessive amounts of these necessary nutrients do not elicit improved antibody production. In fact, it can lead to over-proliferation, nutrient depletion, and by-product accumulation, which results in stunted cultures and low antibody production rates.3,4 Maintaining the minimal amount of nutrients required to facilitate normal cell growth during the entire process can better ensure reliable cell growth and high product yield. Since cell metabolism can change considerably as cells move from growth to production phase,1 spent media analysis is a great tool to determine the levels of nutrients necessary for stable cell growth and antibody production. Many companies, including Irvine Scientific, offer media analysis and development services to help customers achieve the best media compositions and strategies specific for their cells and applications.
In summary, tailoring media to your specific cell line and application allows for maximal production and desired antibody product quality. This cell- and application-specific, fed-batch media development and optimization can be effectively achieved using a combination of paired media survey and “custom” media development optimization using spent media analysis.
References
- Templeton, N., et al. Peak Antibody Production is Associated with Increased Oxidative Metabolism in an Industrially Relevant Fed-Batch CHO Cell Culture. Biotechnology and Bioengineering, 110(7):2013-2024, 2013.
- Kumar, N., et al. Proliferation Control Strategies to Improve Productivity and Survival during CHO-Based Production Culture. Cytotechnology, 53:33-46, 2007.
- Ozturk, S., et al. Chemical Decomposition of Glutamine in Cell Culture Media: Effect of media type, pH, and serum concentration. Biotechnology Progress, 6(2): 121-128, 1990.
- Zeng, A.-P., et al. Determinants and Rate Laws of Growth and Death of Hybridoma Cells in Continuous Culture. Biotechnology and Bioengineering, 57(6):642-654, 1998.
- Li, F., et al. Current Therapeutic Antibody Production and Process Optimization. BioProcessing Journal, 4(5):1-8, 2005.
- Liu, H., et al. Impact of Cell Culture on Recombinant Monoclonal Antibody Product Heterogeneity. Biotechnology Progress, 32(5):1103-1112, 2016.







