We use cookies to make your experience better. To comply with the new e-Privacy directive, we need to ask for your consent to set the cookies. Learn more.
Cohesive Control of Antibody Galactosylation for Improved Antibody Product Quality and Function


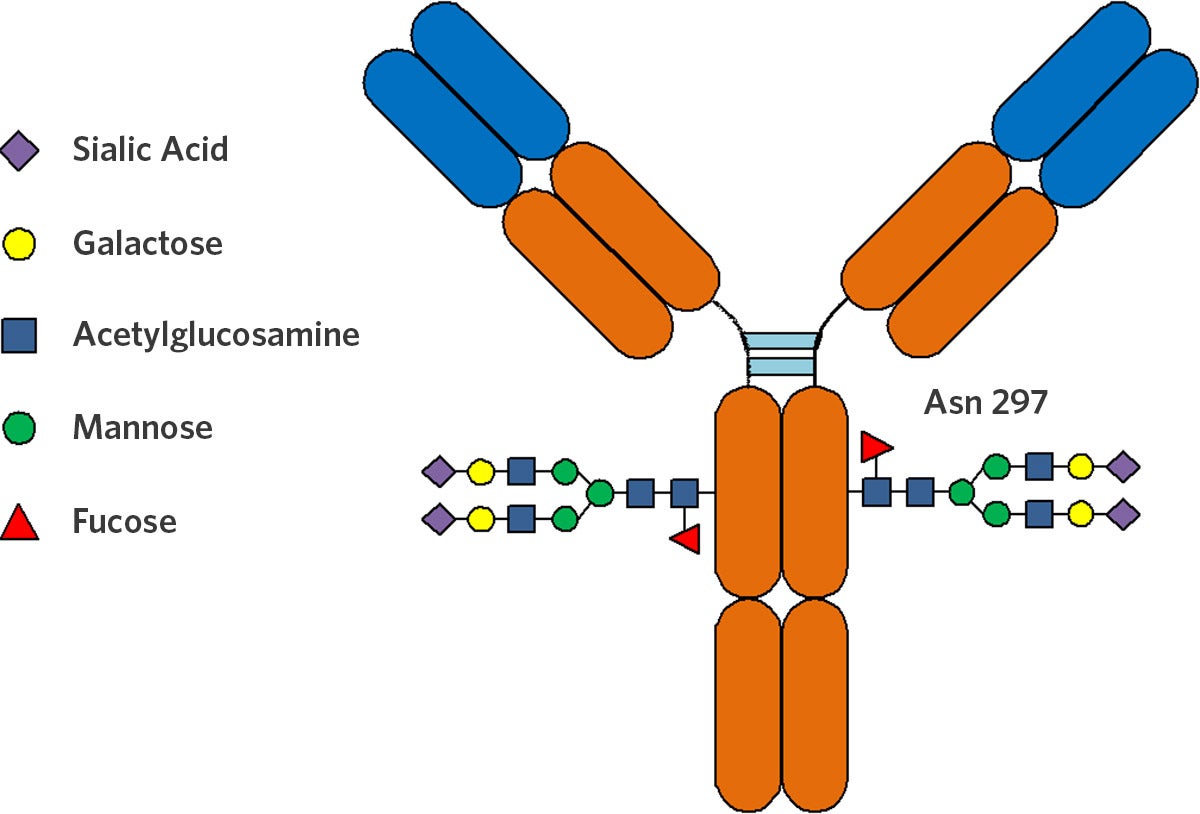
Protein-based therapeutics are dominating the pharmaceutical market due to their high-efficacy in treating complex diseases.1 The demand for antibody (Ab) drugs continues to rise as the global market reaches $84.5 billion USD in 2017 and is expected to reach $114.6 billion by 2022.2
Blockbuster drugs continue to lead not only the Ab drug market but the pharmaceutical market as a whole. As the Ab drug market becomes more aggressive, there is greater pressure for pharmaceutical companies to develop more therapeutically effective Abs. As a result, product quality is becoming more critical in developing the most therapeutically effective Abs.
Glycosylation—or the attachment of sugars to organic molecules—is a critical product quality aspect that has been steadily gaining attention. The type and level of glycosylation can significantly alter Ab binding, function, and therapeutic efficacy of the drug.1,3
Galactosylation—or the glycosylation of galactose—is a key product quality aspect due to both its significant impact on Ab function and the ability to control galactosylation through cell culture medium. The presence of galactose has been found to enhance the complement-dependent cytotoxic activity of rituximab Abs.4 Conversely, the removal of galactose has been found to reduce complement lysis activity.5 Culture medium is a major factor in shaping the galactosylation profile of Abs. Various studies have shown that media composition can modulate Ab galactosylation.6,7,8 Therefore, culture media composition is a simple way to control galactosylation—and ultimately improve Ab product quality and efficacy.
In the following study, Irvine Scientific assessed the ability of media supplements to modulate Ab galactosylation levels and developed an "Optimized Supplement," which significantly increased galactosylation, and resulted in more therapeutically effective Abs.
As an initial media screen, various factors affiliated with galactosylation were evaluated in a Chinese hamster ovary (CHO) fed-batch, design of experiment (DoE) study utilizing BalanCD CHO Growth A and BalanCD CHO Feed 4.
The different DoE conditions had little to no impact on viable cell density and viability as cell growth was similar to the fed-batch control (Figure 1A). Similarly, day 14 titers were minimally affected by the supplements (Figure 1B). The glycan profiles were measured, and selected results are presented in Figure 1C. At 14%, the control with no supplement resulted in the lowest percent galactose. The various DoE conditions resulted in increasing galactose levels from 25% to 50%. DoE analysis with criteria of high galactosylation and minimal impact on cell growth and titer resulted in a supplement composition deemed "Optimized Supplement."
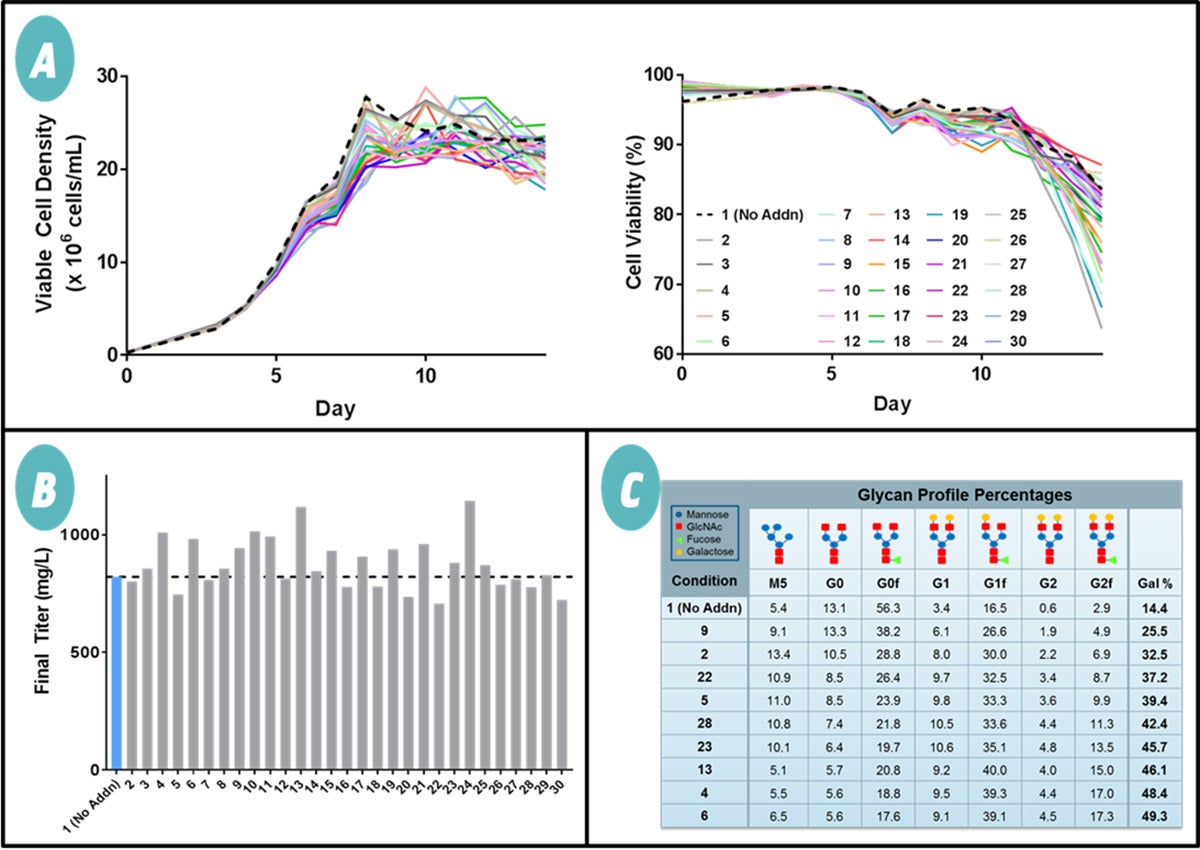
Figure 1. Design of experiment (DoE) study to identify key modulators of galactosylation. Various supplements were added to CHO fed-batch cultures expressing an IgG1 Ab and analyzed for impacts on cell growth, Ab production, and product quality. (A) Viable cell density and cell viability of the 30 DoE fed-batch conditions. Galactose-modulating supplements did not significantly impact cell growth as viable cell densities and cell viabilities were similar to the control, which had no supplementation (Condition 1: No Addn; dashed line). (B) Final Ab titer of the 30 DoE fed-batch conditions. A dashed line is provided to easily compare all the conditions against the control (Condition 1: No Addn; blue bar). Galactose-modulating supplements had a limited effect on Ab titer levels compared to the control. (C) Percentage breakdown of the glycan profiles from a set of the DoE fed-batch conditions. Percent galactose was defined as the number of galactoses divided by the number of possible galactoses. The DoE components were able to increase Ab galactosylation and resulted in a wide range of percent galactose from a low of 14% to a high of 50%.
A fed-batch experiment was conducted with different concentrations of the Optimized Supplement to verify its galactose-enhancing effects. The increasing concentrations of Optimized Supplement did not drastically alter viable cell density, cell viability, or Ab titers throughout the 14-day cultures (Figure 2A & B). Percent galactosylation ranged from 25% to 48% with increasing concentrations of the Optimized Supplement while the control resulted in 20% galactosylation (Figure 2C). The Optimized Supplement did not affect cell growth and Ab titers and succeeded in significantly enhancing Ab galactosylation.
Specific levels of galactosylation can be obtained by first titrating the supplement in the production culture. The supplement has the potential to increase galactosylation with other cell lines and Abs and has the potential to help develop more effective Ab drugs.
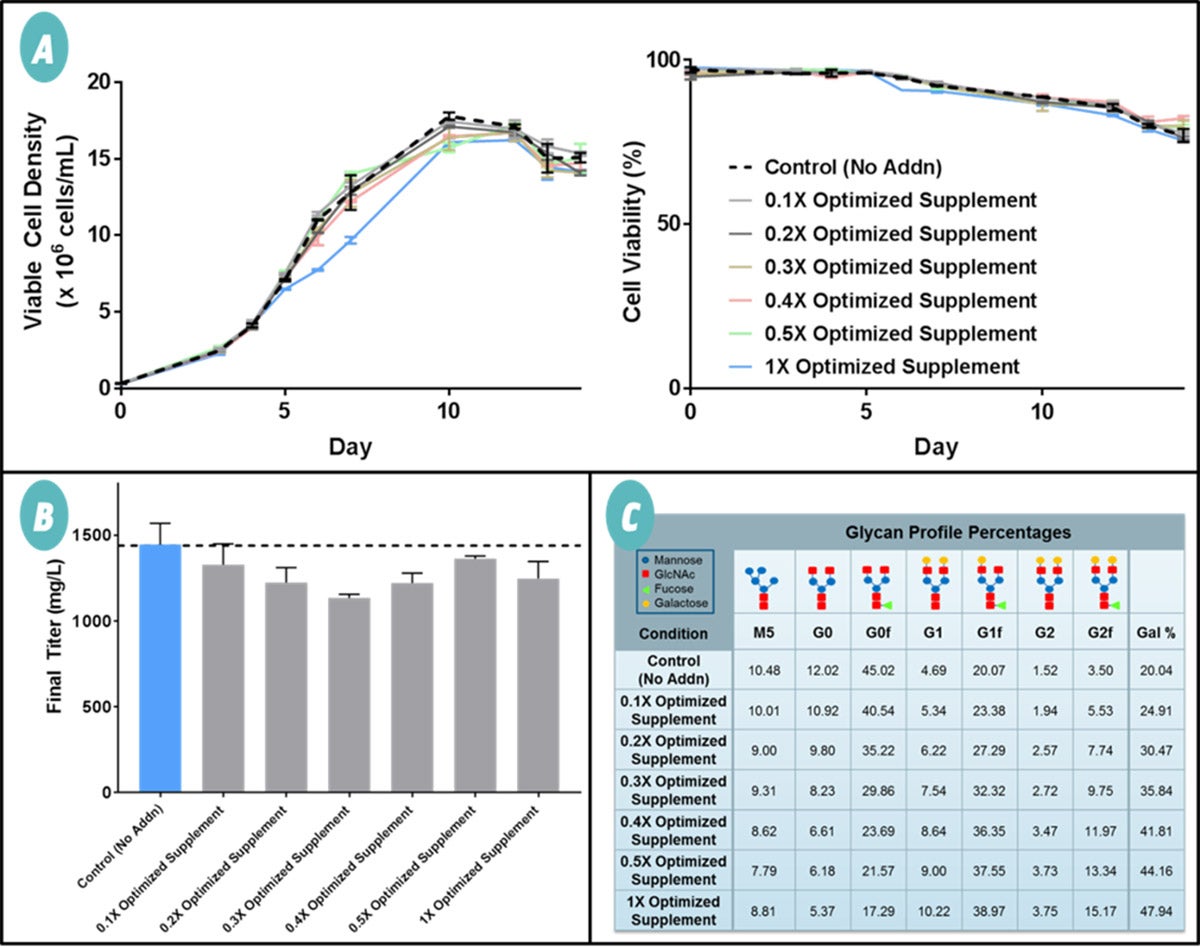
Figure 2 Verification of the Optimized Supplement. Increasing concentrations of the Optimized Supplement were added to CHO fed-batch cultures and analyzed for impacts on cell growth, Ab production, and product quality. (A) Viable cell density and cell viability of the fed-batch cultures. The Optimized Supplement did not significantly impact cell growth as viable cell densities and cell viabilities were similar to the control which had no supplementation (No Addn; dashed line). (B) Final Ab titer of the fed-batch cultures. A dashed line is provided to easily compare all the conditions against the control (Condition 1: No Addn; blue bar). The Optimized Supplement had a limited effect on Ab titer levels compared to the control. (C) Percentage breakdown of the glycan profiles. Percent galactose was defined as the number of galactoses divided by the number of possible galactoses. The Optimized Supplement was able to increase Ab galactosylation and resulted in a wide range of percent galactose from a low of 25% to a high of 48%.
Lastly, the Optimized Supplement was evaluated against a panel of galactose-modulating supplements from other suppliers. The Optimized Supplement resulted in similar viable cell density and cell viability compared to the control fed-batch culture, which had no supplements (Figure 3A).
Supplements from Supplier 1 resulted in similar to half the viable cell density of the control while supplements from Supplier 2 resulted in very low viable cell density and percent viability. Those cultures were ended prematurely since they fell below 70% viability.
All of the supplements, except those from Supplier 2, resulted in Ab titers similar to the control (Figure 3B). Due to the poor growth, and subsequently low titer from Supplier 2's supplement, Supplier 2 was excluded from further evaluation. The remaining Abs were analyzed for glycosylation; the results are presented in Figure 3C.
All the evaluated supplements were able to raise galactosylation; however, only the Optimized Supplement and the 2X Supplier 1 Supplement resulted in over 40% galactosylation. To evaluate the functional activity of the Abs, an in vitro complement-dependent cytotoxicity (CDC) assay was conducted on the highly galactosylated Abs (Figure 3D).
Abs from the Optimized Supplement were more effective than the control Abs and had a significantly lower half-maximal effective concentration (EC50, 1.19 µg/mL) than the control (1.71 µg/mL). Abs from the 2X Supplier 1 Supplement had a similar EC50 to the control—despite having a high percent of galactose. This may be due to the high Man5% generated from 2X Supplier 1's supplement.
The Optimized Supplement outperformed all other suppliers' supplements and resulted in the best overall cell growth, titer, galactosylation, and Ab function.
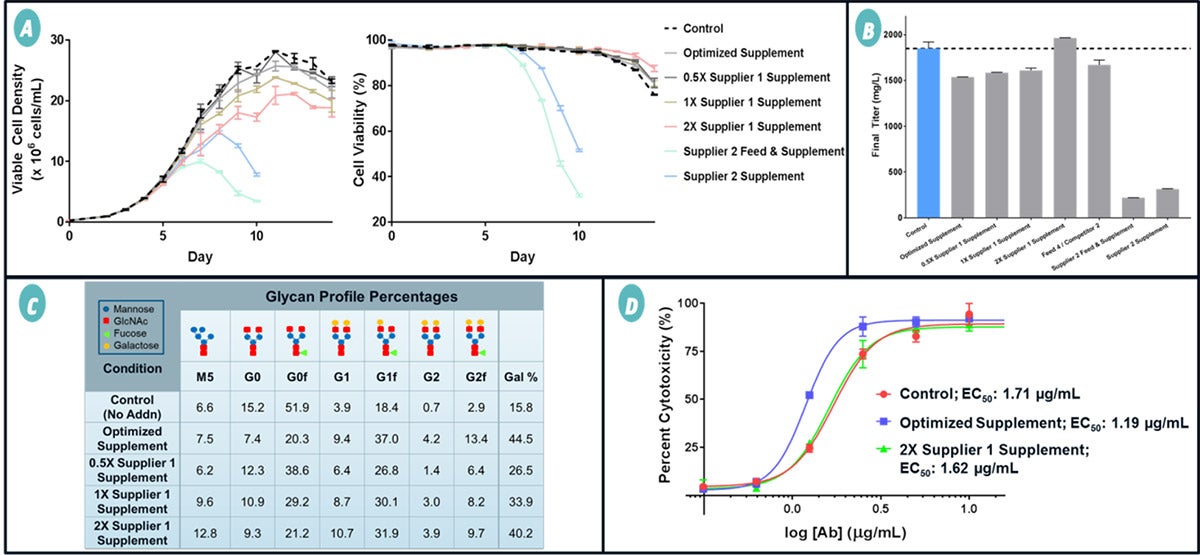

Figure 3 Comparison of the Optimized Supplement to supplements from other suppliers. Supplements were added to CHO fed-batch cultures according to manufacturer's instructions and analyzed for impact on cell growth, Ab production, and functional Ab activity. (A) Viable cell density and cell viability of the cultures with and without galactose-modulating supplements. The Optimized Supplement and Supplier 1 Supplements did not significantly impact cell growth while Supplier 2 Supplements significantly lowered viable cell density and cell viability. (B) Final Ab titer of the cultures. The Optimized Supplement and Supplier 1 Supplements had a limited impact on Ab titer while Supplier 2 Supplements significantly decreased titers due to stunted cell growth and viability. Due to poor growth and titer levels, Supplier 2 was excluded from further studies. (C) Abs from cultures with high viable cell density, viability, and titers were further analyzed for their glycan profiles. The Optimized Supplement and Supplier 1 2X Supplement resulted in Abs with galactose percentage levels of about 45%. The lower concentrations of Supplier 1's Supplements resulted in lower galactose percentages and were excluded from further studies. (D) Abs with high galactosylation were further evaluated on their therapeutic effect and ability to induce CDC in vitro. The anti-CD20 Abs were incubated with Daudi lymphoblast cells and normal human complement serum. After enough time had elapsed for complement cascade lysis to occur, cell death was measured by a cytotoxicity assay. Increased galactosylation by the Optimized Supplement resulted in more therapeutic Abs with lower EC50 values than the Control. Conversely, the high galactosylation from the Supplier 1 2X Supplement did not result in as low of an EC50 value compared to the Abs from the Optimized Supplement.
Through DoE screening and analysis, Irvine Scientific has developed an Optimized Supplement designed to increase the galactosylation level of Abs. This supplement had minimal effect on cell growth and titer and was able to increase galactosylation by 3.5-fold over control Abs. When compared with galactose-enhancing supplements from other suppliers, the Optimized Supplement resulted in better cell growth, higher titers, increased galactosylation, and more potent Abs.
Overall, Irvine Scientific can optimize media for increased galactosylation, as well as measure glycosylation levels, and evaluate the therapeutic function of Abs. This allows for cohesive control of product quality that can be utilized to improve Ab drug efficacy by further understanding the relationship between protein structure and function. Nevertheless, each Ab is unique, and one culture process may not produce the same product quality for every Ab. Therefore, each Ab and application pair may need its own media optimization process to produce the most effective Abs.
Through our media development and optimization services, Irvine Scientific can help you develop a custom supplement to help produce Abs with optimal galactosylation levels and maximal efficacy in your specific process.
References:
- Batra J, Rathore AS. Glycosylation of monoclonal antibody products: Current status and future prospects. Biotechnology Progress. 2016;32(5):1091-1102. doi:10.1002/btpr.2366.
- Dewan, Shalini. Antibody drugs: Technologies and global markets. BCC Research 2017.
- Hossler P, Khattak SF, Li ZJ. Optimal and consistent protein glycosylation in mammalian cell culture. Glycobiology 2009, 19: 936-949.
- Hodoniczky J, Zheng YZ, James DC. Control of recombinant monoclonal antibody effector functions by fc N-glycan remodeling in vitro. Biotechnology Progress 2005, 21: 1644-1652.
- Boyd PN, Lines AC, Patel AK. The effect of the removal of sialic acid, galactose and total carbohydrate on the functional activity of Campath-1H. Molecular Immunology 1995, 32 (17/18): 1311-1318.
- Boniface R, Shageman J, Sanderson B, Gillmesiter M, Varela-Rohena A, Yan J, Tennico Y, Barrett S, Setterquist R, Gorfien S. Profiling of glycosylation gene expression in CHO fed-batch cultures in response to glycosylation-enhancing medium components. BMC Proceedings 2013, 7 (6): P99.
- Grainger RK, James DC: CHO cell line specific prediction and control of recombinant monoclonal antibody N-glycosylation. Biotechnology and Bioengineering 2013, 110 (11): 2970-2983.
- Gramer MJ, Eckblad JJ, Donahue R, Brown J, Shultz C, Vickerman K, Priem P, van den Bremer ETJ, Gerritsen J, van Berkel PHC. Modulation of antibody galactosylation through feeding of uridine, manganese chloride, and galactose. Biotechnology and Bioengineering 2011, 108 (7): 1591-1602.







