We use cookies to make your experience better. To comply with the new e-Privacy directive, we need to ask for your consent to set the cookies. Learn more.
Unparalleled Quality System
As worldwide cell culture media providers, FUJIFILM Irvine Scientific is regulated and adheres to both FDA and ISO regulations and conducts regular audits of suppliers and raw materials. Our ISO 13485:2016 certified Quality System uses a risk-based approach to remove potential risks and reliably deliver the highest quality products. We adhere to stringent policies and standard operating procedures from the initial evaluation and qualification of suppliers to our extensive testing and qualifying of raw materials, including our proprietary genetic mouse embryo assay (MEGA) to identify embryotoxicity at an epigenetic level and provide a more sensitive, relevant testing method of critical raw materials than 1-cell MEA alone. Internal audits are also conducted at planned intervals to ensure our Quality System is effectively implemented and maintained to safeguard the consistency of supply and performance.
Quality and performance by design
Each ART product is designed to meet customer specifications and exceed performance expectations. Our design control process is an aspect of 21 CFR 820 Quality System Regulation that incorporates user needs, a review of each design process point, and extensive input and output verification to demonstrate proper documentation and traceability. With our in-depth knowledge, experience, and expertise within the ART industry, we lead by example with extensive testing, innovative quality control measures, and post-market clinical follow-up.
Global regulatory compliance
FUJIFILM Irvine Scientific provides extensive regulatory support to ensure the qualification and compliance of products used in the IVF clinic. This encompasses strict manufacturing qualifications and clearances to affirm the optimal performance and safety of our products.
- Guideline for Manufacture of In Vitro Diagnostic Products and the Good Manufacturing Practices (GMP's) for Medical Devices
- FDA Cleared; FDA QSR
- European Medical Devices Directive (93/42/EEC); CE Mark*
- 21 CFR 820 Quality Systems Regulations (US FDA)
- ISO 13485:2016
- Medical Device Single Audit Program (MDSAP)
* With the exception of a small subset of ancillary products.
Rigorous raw material program
At FUJIFILM Irvine Scientific, we understand that to deliver quality products, we must start with the most qualified raw materials. Our robust raw materials program meets and exceeds regulatory requirements for the sourcing, testing, and documentation of raw materials. We begin by qualifying and auditing every supplier and conduct continuous monitoring to ensure they meet our standards.
- Risk-based approach
- Confirmation of supplier definitions
- Customer testing
- QC testing and pilot batch testing
- Document verification
MEGA assures consistency and safety
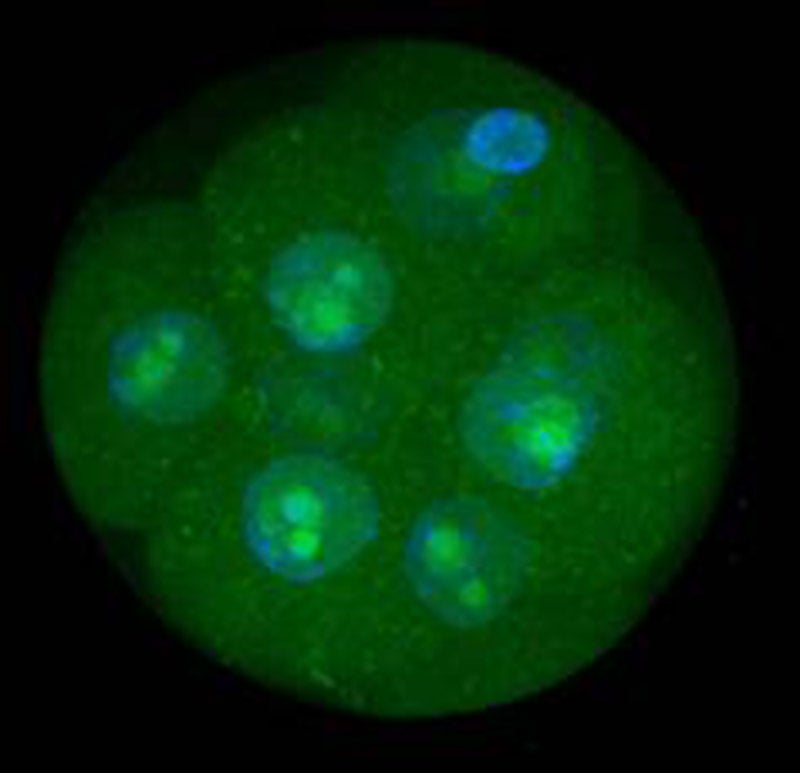

Identifying embryotoxicity within oil and human serum albumin for IVF procedures requires more sensitive testing than the standard mouse embryo assay can detect. FUJIFILM Irvine Scientific developed the MEGA assay to screen oil and human serum albumin using a unique fluorescence-based detection of developmentally regular gene expression to assess embryos through the development stages—including at the earliest stages of growth. The result is a bioassay that is more sensitive to embryotoxic materials and provides more functional information at an earlier stage of development while ensuring safety and quality control of media used in the laboratory.
- Enables analysis of pre-implantation embryo development and health by gene expression in addition to morphology
- Increased sensitivity for detecting embryotoxicity in critical raw materials before manufacture
- Assessment of efficacy at early stages of development
Extensive product testing
FUJIFILM Irvine Scientific performs a rigorous panel of tests to confirm our products' quality, safety, and lot-to-lot consistency. Results are provided in lot-specific Certificates of Analysis.
- 1-cell MEA
- Appearance
- Endotoxin
- Function
- Human Sperm Survival assay
- Osmolality
- pH
- Sterility
- Visual inspection
WFI Quality Water
Water purity is crucial in the IVF clinic and in manufacturing media. FUJIFILM Irvine Scientific produces WFI quality water to manufacture our products using a pre-treatment system, vapor compression still, and WFI distribution loop, all of which are continuously monitored to ensure quality and safety. This enables us to control all aspects of our product quality.
- Our WFI water system is validated according to FDA and USP guidelines
- Bottled WFI Quality Water undergoes strict Quality Control and MEA testing
Committed to quality service and support
For FUJIFILM Irvine Scientific, quality goes beyond the purchase of our products. We support customers by ensuring that our products and their protocols are optimized to provide the best results possible. We regularly survey our customers to confirm their satisfaction, deliver optimal product performance, and provide them with support and consultation from our Technical Application Scientists on the proper use of our products in their clinics. Together with our Quality System and highly experienced team, our commitment to quality service and support helps our customers achieve successful results for their patients.
Extensive product testing
FUJIFILM Irvine Scientific performs a rigorous panel of tests to confirm our products' quality, safety, and lot-to-lot consistency. Results are provided in lot-specific Certificates of Analysis.
- 1-cell MEA
- Appearance
- Endotoxin
- Function
- Human Sperm Survival assay
- Osmolality
- pH
- Sterility
- Visual inspection
Committed to quality service and support
For FUJIFILM Irvine Scientific, quality goes beyond the purchase of our products. We support customers by ensuring that our products and their protocols are optimized to provide the best results possible. We regularly survey our customers to confirm their satisfaction, deliver optimal product performance, and provide them with support and consultation from our Technical Application Scientists on the proper use of our products in their clinics. Together with our Quality System and highly experienced team, our commitment to quality service and support helps our customers achieve successful results for their patients.
WFI Quality Water


Water purity is crucial in the IVF clinic and in manufacturing media. FUJIFILM Irvine Scientific produces WFI quality water to manufacture our products using a pre-treatment system, vapor compression still, and WFI distribution loop, all of which are continuously monitored to ensure quality and safety. This enables us to control all aspects of our product quality.
- Our WFI water system is validated according to FDA and USP guidelines
- Bottled WFI Quality Water undergoes strict Quality Control and MEA testing

