We use cookies to make your experience better. To comply with the new e-Privacy directive, we need to ask for your consent to set the cookies. Learn more.
Protocol for Mesenchymal Stem Cell Expansion
Mesenchymal stem cells(MSCs) are currently widely used in clinical trials for cell therapy and regenerative medicine applications. Whether MSCs are used in basic research or in translational studies, establishing the optimal mesenchymal stem cell expansion method and best performing medium allows for more reliable end results.
The following protocol outlines steps for mesenchymal stem cell expansion using PRIME-XV MSC Expansion SFM. This medium is manufactured under cGMP conditions and comes completely formulated and ready for use. For more information on this medium, please refer to the product insert.
Materials
- Phosphate Buffered Solution (PBS) (Irvine Scientific, Catalog #9236)
- Culture vessels (T-25 or T-75)
- PRIME-XV Human Fibronectin (Irvine Scientific, Catalog #31002) or PRIME-XV MatrlS F (Irvine Scientific, Catalog #31001)
- PRIME-XV MSC Expansion SFM (Irvine Scientific, Catalog #91135)
- Frozen vial of mesenchymal stem cells
- 15 mL conical, centrifuge tubes
- MSC-cultured vessel (from Protocol: Thawing Mesenchymal Stem Cells)
- Cell Counter
- TrypLE Express
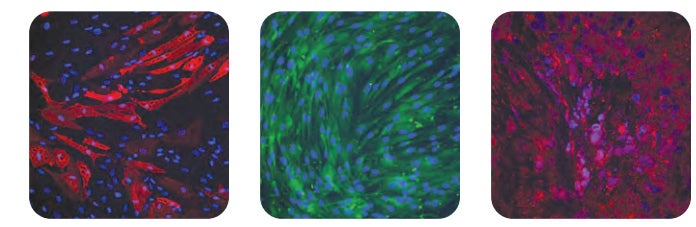

Multi-lineage differentiation potential was maintained in human bone marrow-derived MSCs after culturing in PRIME-XV MSC Expansion SFM, as seen in immunofluorescence analysis of FABP-4 (left), Osteocalcin (middle), and Aggrecan (right), representing the adipogenic, osteogenic, and chondrogenic lineages, respectively. Nuclei were counterstained with DAPI (blue).
Protocols
The following mesenchymal stem cell expansion protocols can be found below:
- Coating Culture Vessels
- Thawing Mesenchymal Stem Cells
- Expansion and Subculture of Human Mesenchymal Stem Cells
For materials and protocols on analysis and isolation, please visit Mesenchymal Stem Cell – Immune Modulation and Mesenchymal Stem Cell Isolation from Adipose, Cord Blood, and Umbilical Cord
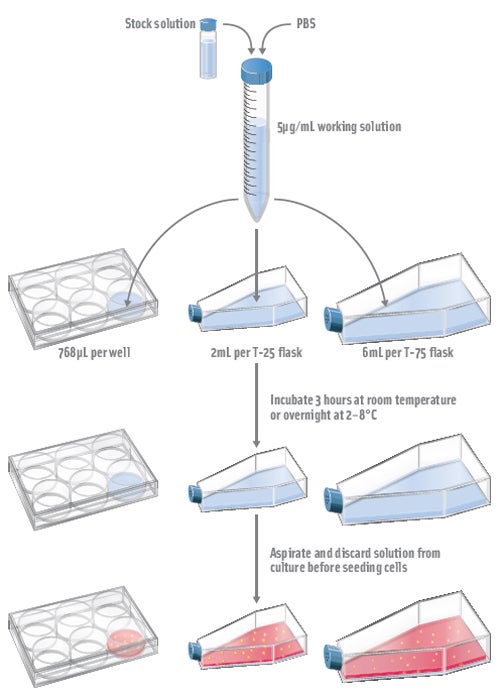
Coating Culture Vessels
Extracellular attachment substrate recommended for optimal performance:
- Prepare a 5μg/mL working solution in PBS of PRIME-XV MatrIS F or PRIME-XV Human Fibronectin
- Add the diluted product to culture vessel at a final volume per surface area of 0.08mL/cm2. Refer to the table below for appropriate culture volume usage.
Note: Reconstitute PRIME-XV Human Fibronectin to 100μg/mL stock solution in 1X Dulbecco’s Phosphate Buffered Saline (PBS) before use.
Culture Vessel
Surface Area (cm2)
Volume of 5μg/mL PRIME-XV Attachment Substrate
6-well plate
9.6
768 µL/well
T-25 flask
25
2 mL/flask
T-75 flask
75
6 mL/flask
- Incubate culture vessels at room temperature for 3 hours or overnight at 2-8°C. The culture vessel must be sealed with Parafilm® to avoid drying if stored at 2-8°C overnight. It is recommended to coat culture vessels the day of use or the day before use.
- Aspirate out and discard diluted solution from culture vessels before the addition of mesenchymal stem cells.

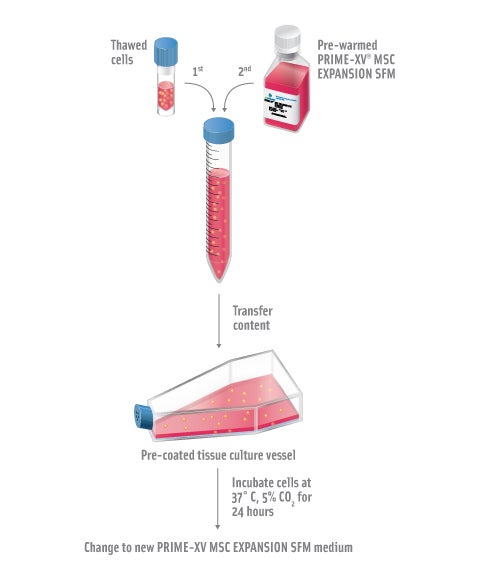
Thawing Mesenchymal Stem Cells
-
Pre-coat the tissue culture vessel with 5µg/mL of PRIME-XV MatrIS F or PRIME-XV Human Fibronectin for 3 hours at room temperature or overnight at 2-8°C. See “Coating Culture Vessels.”
-
Pre-warm PRIME-XV MSC Expansion SFM to 37°C for no more than 30 minutes. Repeated warming of medium may reduce product performance.
-
Rapidly thaw frozen vial of mesenchymal stem cells in a 37°C water bath. Sample should be thawed with gentle swirling until all visible ice has melted. Thaw time for a 1mL sample in a cryovial is approximately 3 minutes.
Note : DO NOT allow sample to warm above chilled temperatures (0-10°C). Cryovials should be cool to the touch when removed from the water bath. -
Pipette the entire content of the cryovial into a 15mL conical tube. Carefully add 5-10mL of pre-warmed PRIME-XV MSC Expansion SFM at an approximate rate of 3-5 drops per 10 seconds and gently swirl after each addition.
-
Transfer the entire content of the conical tube into a pre-coated tissue culture vessel.
-
Incubate the cells at 37°C, 5% CO2 incubator.
-
Aspirate off media and feed the cells with pre-warmed PRIME-XV MSC Expansion SFM 24 hours post-thaw
-
Remove and discard spent media every two days, and feed cells with pre-warmed PRIME-XV MSC Expansion SFM. Subculture when cells reach 80-90% confluence. Do not allow the cultures to become completely or over confluent. Subculturing under suboptimal conditions may affect product performance.

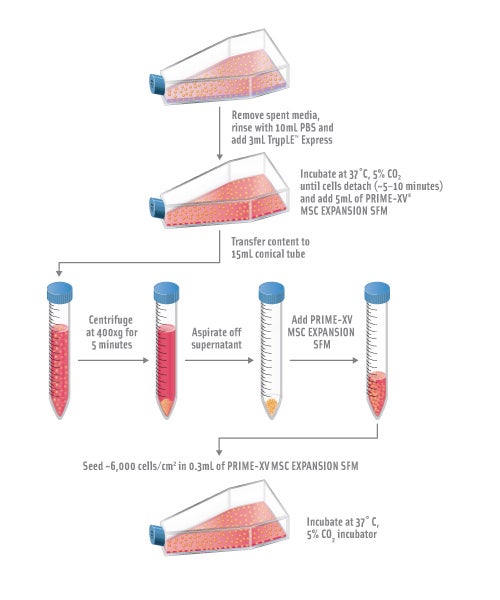
Expansion and Subculture of Human Mesenchymal Stem Cells
-
Pre-coat the tissue culture vessel with 5µg/mL of PRIME-XV MatrIS F or PRIME-XV Human Fibronectin for 3 hours at room temperature or overnight at 2-8°C. See “Coating Culture Vessels.”
-
Pre-warm PRIME-XV MSC Expansion SFM to 37°C for no more than 30 minutes. Repeated warming of medium may reduce product performance.
-
Remove spent media from T-75 flask culture and gently rinse cells once with 10mL of PBS for each T-75 flask.
-
Add 3mL of room temperature TrypLE Express to each T-75 flask, and tilt the flask in all directions to disperse the TrypLE™ Express evenly over the cells.
-
Incubate the cells at 37°C, 5% CO2 incubator. Monitoring periodically for cell detachment by observing the cells under the microscope. Cells will start to round and detach. Tap the side of the flask to aid the detachment of the cells and return culture to the incubator. Repeat the above process until at least 90% of cells are fully detached. This process takes approximately 5-10 minutes.
-
Add 5mL of PRIME-XV MSC Expansion SFM to the flask. Disperse the cells by pipetting the media over the entire growing surface of the flask, and transfer the contents to a 15mL conical tube.
-
Centrifuge cells down at 400xg for 5 minutes. Aspirate off supernatant.
-
Resuspend the cell pellet in a small amount of pre-warmed PRIME-XV MSC Expansion SFM and count the cells with a cell counter.
-
Resuspend 4.5-5.0 x 105 cells into 20mL of pre-warmed PRIME-XV MSC Expansion SFM for each pre-coated T-75 flask.
Note: It is recommended to seed cells at approximately 6,000 cells/cm2 in 0.3mL of media for 2-dimensional pre-coated culture vessels. -
Gently aspirate off PRIME-XV attachment substrate solution from the flask and slowly add the cell suspension to a T-75 flask. Avoid scraping the coated surface when aspirating. Incubate the cells at 37°C, 5% CO2 incubator.
-
Remove and discard spent media. Feed the cells with pre-warmed PRIME-XV MSC Expansion SFM every 2 days.
* TrypLE is a registered trademark of Life Technologies.